June 1 – 5, 2024
American Transplant Congress (ATC)
Philadelphia, PA
Meet us at Booth #720 to discover more about how you can help your transplant patients
June 5, 2024 - 6 pm EST
Advanced Strategies for CMV Management after SOT
Live Online Event
For the full scientific program, panel member list and registration click here

CMV infection remains one of the most common complications for solid organ transplant recipients1 and is still considered “the troll of transplantation."2
As an immune globulin, CYTOGAM® offers a complementary mechanism of action to antiviral agents, working outside the cell, thus adding enhanced protection against CMV post-transplant.3,4,5
References
- Haidar G, Boeckh M, Singh N. Cytomegalovirus infection in solid organ and hematopoietic cell transplantation: state of the evidence. J Infect Dis. 2020;221(S1):S23-S31. doi: 10.1093/infdis/jiz454
- Stern A, Papnicolaou GA. CMV prevention and treatment in transplantation: what’s new in 2019. Curr Infect Dis Rep. 2019; 21(11): 45. doi:10.1007/s11908-019-0699-0
- Grossi PA, Mohacsi P, Szabolcs Z, Potena L. Cytomegalovirus immunoglobulin after thoracic transplantation: an overview. Transplantation. 2016; 100: S1-S4
- Carbone J. The immunology of posttransplant CMV infection: potential effect of CMV immunoglobulins on distinct components of the immune response to CMV. Transplantation. 2016;100: S11-S18
- Valantine HA, Luikart H, Doyle R, et al. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin versus ganciclovir alone. Transplantation. 2001;72(10):1647-1652
Resources
Mechanism of Action Video
2025 CEoT Product Theater - Innovation Approaches to CMV Management
Transforming the Role of Cytogam
Part 1 - Speaker: Marie M. Budev DO, MPH FCCP
Part 2 - Speaker: Winston Ally, PharmD
ATC 2024 Tufts Poster - Introduction VideoNew
2024 CEoT Product Theater
Breakthroughs in Hyperimmune Therapy
Anti-CMV Prophylaxis Strategies and Immune Response (in Lung Transplantation)

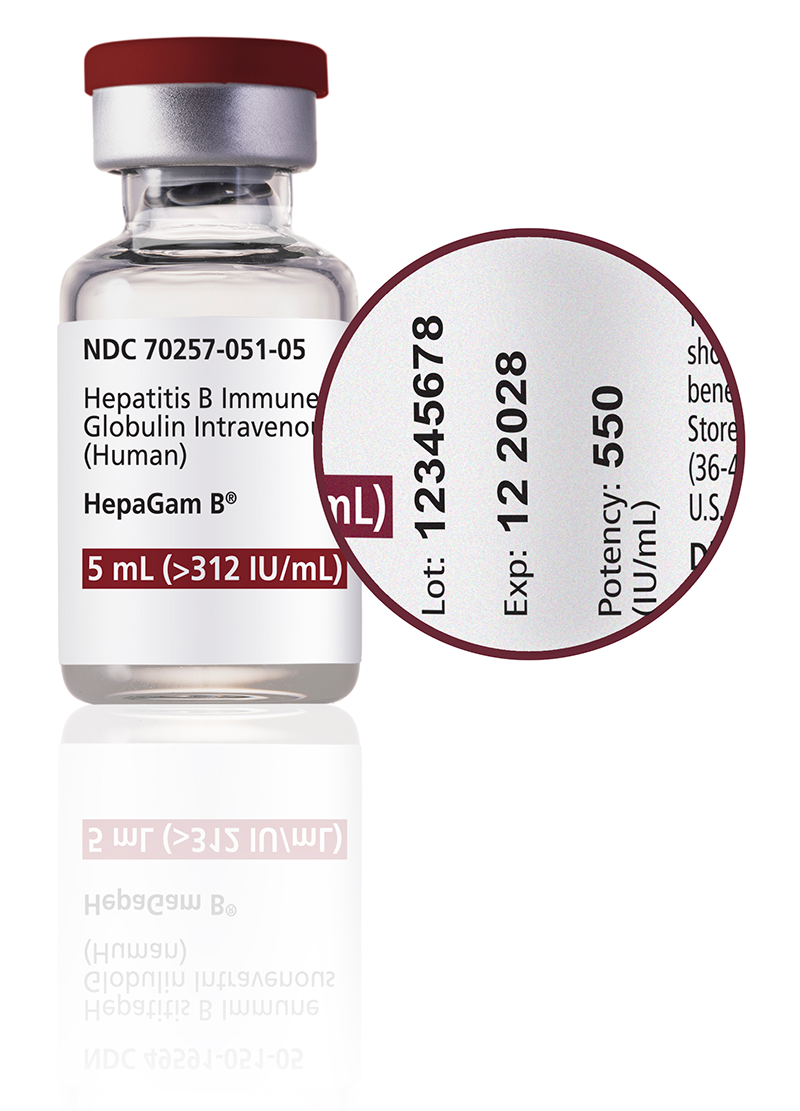
HEPAGAM B is the only Hepatitis B Immune Globulin (HBIG):
- Approved for post-transplant prophylaxis of hepatitis B in liver transplants.1
- Proven to be safe and effective in preventing HBV recurrence following liver transplants2
- With actual potency indicated on each vial to ensure high dosing accuracy1
References
- Kamada corporate presentation, September 2022
- Terrault NA, Kilic M, Karademir S, et al. HepaGam B after liver transplant in patients with hepatitis B virus. Available at: https://www.researchgate.net/publication/267792129_Orthotopic_HepaGam_B_After_Liver_Transplant_in_Patients_with_Hepatitis_B_Virus. Accessed July 27, 2022

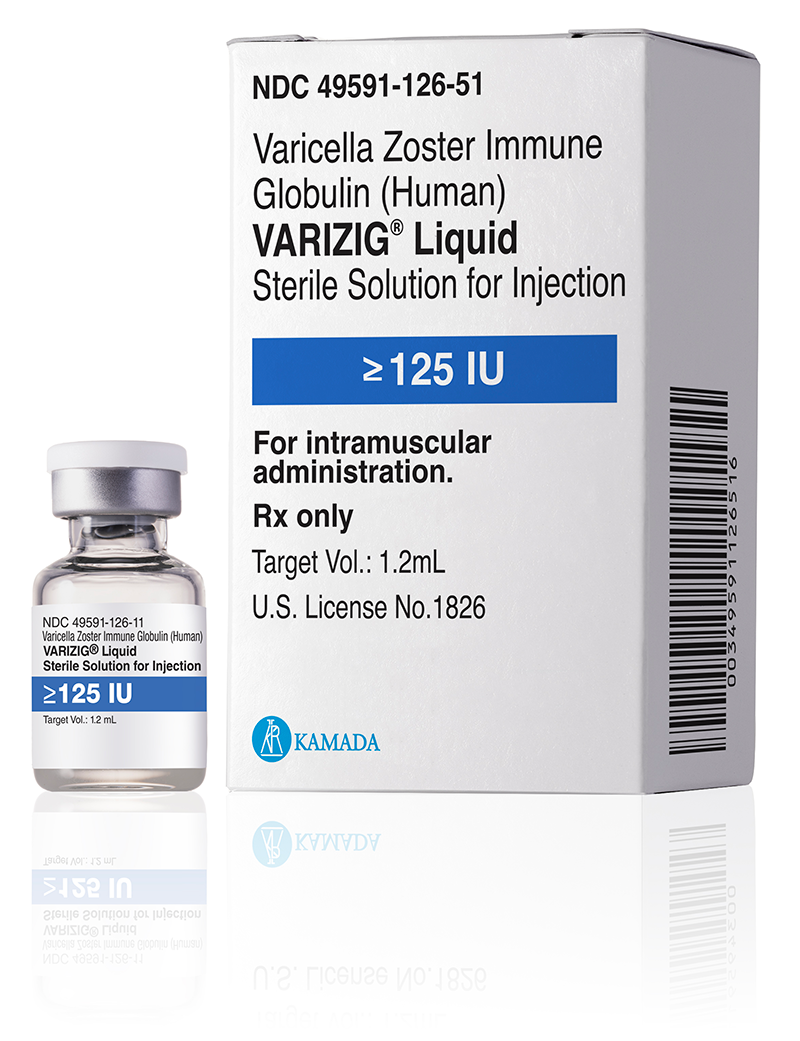
Varicella-zoster virus (VZV) infection remains a significant concern for transplant patients without sufficient VZV immunity1.
VARIZIG® is recommended as first-line treatment by leading guidelines for VZV post-exposure prophylaxis, to effectively reduce disease severity and complications after exposure of a high-risk individual to VZV1-3.
References
- Varicella Zoster virus in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice, 2019.
- The CDC updated Recommendations for Use of VARIZIG - United States, 2013.
- Red-Book: 2021–2024 Report of the Committee on Infectious Diseases.